
Today’s consumers take for granted the ability of the electronics industry to squeeze more functions into ever smaller, more portable devices. Mobile phones double as cameras and are now adding flash features; still cameras shoot video; laptop computers can do pretty much anything that can be achieved on the desktop, including driving power-hungry peripherals.
This growing array of functions poses a problem, though, for the systems that are used to provide the power – primarily the battery. And, whilst great strides have taken place in cutting active and standby power consumption of electronic devices, it is the changing nature of the functions that now represents the greatest challenge. Products such as high data-rate GPRS transmitters and cameras with electronic flash and auto-focus drives require significant instantaneous pulses of power that are difficult to deliver from a portable power source.
At the same time as power requirements have become more exacting, product designers have worked to make it possible to use mainstream battery technology in portable devices. Primarily, this means moving to what remains the most common, low-cost form of battery power: the alkaline cell, typified by the familiar AA battery.
Alkaline batteries have many advantages:
* They are readily available at reasonable cost.
* Consumers are familiar with the technology and confident in handling them.
* They provide high energy density and long life.
* They are easy to design in, are long established and hence well understood by engineers.

High pulse-power applications take a toll on batteries
Alkaline batteries also have some weaknesses in applications with high pulse power. In particular, their internal resistance (expressed as equivalent series resistance or ESR) causes an internal voltage drop when current is drawn. This means that their ability to deliver short spikes of current is relatively poor. This phenomenon worsens in cold conditions and as the battery discharges – and in high pulse power applications the system typically shuts down due to the resulting drop in the output voltage during the pulse; worse, in these high pulse power applications the battery typically will have an appreciable amount of unused energy left at the time of low-voltage shutdown.
In a typical case, a digital still camera driven by two AA batteries might draw a peak power of 3 W and current of 1,5 A during the auto focus phase of its operation. Such a drive function might last around 0,4 seconds, during which time the battery voltage would drop around 300 mV for a new battery, but the voltage drop will increase to as much as 600 mV when the battery reaches approximately 50% charge level. Results will vary from battery to battery and across manufacturers.

Can capacitors support batteries with short-term power boosts?
Such challenges have aroused much interest in technologies that can be used alongside conventional batteries to provide a short-term boost in current delivering capabilities. In principle, such effects could be achieved by charging and discharging capacitors, which have a much higher power density than batteries.
Unfortunately, the energy density of a conventional capacitor is inadequate for the purpose. They are useful for supplying short power pulses on the order of milliseconds, but simply cannot store sufficient charge to solve problems in applications such as an auto-focus drive, which, as the above example illustrates, require storage of a substantial fraction of a Coulomb of charge.
Exploring the benefits of supercapacitors
Attention has turned to electrochemical double-layer capacitors (EDLCs), otherwise known as supercapacitors. Such devices use highly porous activated carbon materials to realise internal structures with large surface areas. The carbon materials in question provide an effective surface area per gram for charge storage of over 2000 square metres. This is used to form the equivalent of the ‘plates’ of a traditional capacitor. The dielectric portion of the design is formed by a molecule-thin layer of electrolyte, containing ions from a salt combined with a solvent.
As a result, the entire structure operates at the atomic scale, with an electrical interface spacing of the order of 10-10m. The breakdown voltage of the electrolyte controls the operating voltage of the device, and ranges from 1,1 V to 2,5 to 3 V depending on the particular chemistry used in the device.
Supercapacitors use these design principles to deliver dramatically higher energy storage capability than conventional capacitors. They have in fact been known and produced for many years. However, most products have been designed for applications such as memory backup that need very low currents. As a result, another of their characteristics – high ESR (up to 100 Ω in many cases) – has not been a design issue.
Supercapacitors with low ESR result in high power densities
By implementing advanced technologies such as the use of polymer electrolytes, vendors such as CAP-XX have produced a type of device with very low ESR: figures as low as a few milliohms are achievable, which results in power densities that are much higher than most batteries. These supercapacitors have energy efficiencies that rarely fall below 90%, can be charged extremely quickly and safely, achieve capacitance values of up to 2,8 F, and can survive hundreds of thousands of charge-discharge cycles without significant degradation in performance.
Electrically, such devices behave in their discharge phase very much like capacitors. They have physical rather than chemical charge storage so that virtually all of the energy stored in a supercapacitor can be practically recovered, whereas, even with modern alkaline designs that are designed to degrade sharply and fail suddenly at the end of their life, an appreciable proportion of the energy in a battery is irretrievable.
Examples of extended battery life in supercapacitor-equipped devices
In the previously cited camera auto-focus drive, for instance, an appropriate device connected in parallel with the battery can supply most of the pulse current requirements, with the percentage increasing as the battery ages. This cuts the peak battery current to 640 mA, from 1,5 A, and hence reduces the battery voltage droop by an order of magnitude, to 44 mV.
What this equates to in real terms is greatly extended battery life – the supercapacitor-equipped camera can take 31 shots on a single pair of AA batteries, against 22 shots for the circuit without supercapacitor support. The supercapacitor-equipped system draws approximately the same battery current each time a photo is taken, minimising the deterioration of the battery.
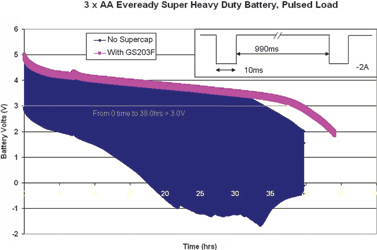
Trials undertaken by CAP-XX on a range of battery brands with higher current pulses such as found in GSM/GPRS applications have produced even more dramatic results refer to the accompanying figures.
The testing procedure involved drawing 2 A, 10 ms pulses from an arrangement of three AA batteries connected in series, at a rate of one pulse per second. The experimenters then measured the time taken for the battery voltage to drop below 3 V. Circuits equipped with a supercapacitor exhibited up to four times the battery life of those using batteries only. This increase in useful life opens up many new applications for alkaline batteries in GSM/GPRS applications such as asset tracking devices for containers where having a long-life, low-cost battery that can be easily replaced is an advantage for the user.
Other considerations when designing with supercapacitors
Designers working with supercapacitors may need to incorporate a few changes to their basic circuits. The devices naturally tend to draw high current at power-on, which could require the use of some kind of simple current limiting circuit, but in most alkaline battery applications the battery will self-limit with no damage. Also, the existing low-voltage lock-out function should be designed with the use of a supercapacitor in mind.
As long as the engineer observes these simple design rules, supercapacitors can allow the use of standard alkaline batteries in many applications from which they were previously excluded. As designers of portable products cram more power-hungry functions into their designs, such supplementary power solutions may provide the answer to many cost, size and weight problems.
For more information contact Seven Labs Technology, +27 (0)11 234 7319, [email protected]
© Technews Publishing (Pty) Ltd | All Rights Reserved